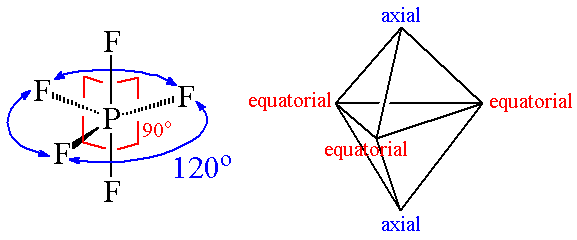Here is a discussion about VSEPR (Valence Shell Electron Pair Repulsion) Theory and its applications. Questions are asked and it can be downloaded from the link given at the end of this discussion.
In the year 1957 Gillespie developed a VSEPR theory to explain molecular shapes and bond angles more accurately. The main postulates of this theory are:
1. The shape of a molecule can be predicted from the number and type of valence shell electron pairs around the central atom
2. Electron pairs around a central atom must stay as far as possible to minimize repulsion.
3. Lone pairs occupy more space around the central atom than the bonded electron pairs.
The order of repulsion between different types of electron pairs is as follows:
Lone pair - Lone pair > Lone Pair - Bond pair > Bond pair - Bond pair
4. The magnitude of repulsion between bond pair's of electrons depend on the electro negativity difference between central atom and bonded atoms.
5. The repulsion order in relation to the bonds are as follows:
double bond-double bond > double bond-single bond > single bond-single bond.
Applications of VSEPR theory to simple molecules:
If there are two electron pairs around the central atom, the only way to keep them as far apart as possible is to arrange them at an angle of 180° to each other. The molecule in such a case will adopt linear geometry. Similarly, the molecule forms trigonal planar geometry for three electron pairs around the central atom, and for four electron pairs around the central atom, the molecule adopts tetrahedral geometry.
Molecules with only bond pairs adopt the above geometry depending upon the number of bonded atoms present, since the above arrangement gives least repulsion among electron pairs and maximum stability.
Distortions from a molecular arrangement arises only when lone pairs are present in a molecule.
Let us take two examples NH3 and H2O to explain the effect of lone pairs in molecular arrangement and compare them with methane.
Shape of NH3 molecule:
We Know that ammonia contains 3 bp and 1 lp ( calculation of bond pair and lone pair of molecule is discussed below)
As lone pair-bond pair repulsion is more than bond pair-bond pair repulsion, the presence of lone pair in ammonia pushes the bond pair from regular tetrahedral geometry. ( ie,lone pair occupy more space) Hence the bond angle decreases from 109.5° to 107°. The geometry of ammonia molecule is also considered as pyramidal.

Shape of H2O molecule:
We Know that water contains 2 bp and 2 lp ( calculation of bond pair and lone pair of molecule is discussed below)
As the repulsive force between lone pair-lone pair is greater lone pair-bond pair repulsion, the presence of two lone pairs in water pushes the bond pair more strongly from regular tetrahedral geometry. ( ie,lone pair occupy more space) Hence the bond angle decreases from 109.5° to 104°. The geometry of water molecule is also considered as bent or angular.

Shape of CH4 molecule:
We Know that methane contains 4 bp. These four electron pairs, trying to remain as far apart as possible, adopt tetrahedral structure. In this geometry, all the H-C-H bond angles are of 109.5°.

Here is a simple calculation used to predict the number of electron pairs both bonded and lone pairs.
Ex. 1: CH4
Step -1
Number of valence electron of carbon (central atom) = 4
Number of bonded atoms (four hydrogen) = 4
Total = 8
Step - 2
divide this by 2 (8/2) = 4
so there must be 4 electron pairs in this molecule and all are bonded with four hydrogen. (ie, no lone pair is present).
Hence, this molecule posses a regular geometry (no distortion) tetrahedral.
Ex. 2: NH3
Step -1
Number of valence electron of nitrogen (central atom) = 5
Number of bonded atoms (three hydrogen) = 3
Total = 8
Step - 2
divide this by 2 (8/2) = 4
so there must be 4 electron pairs in this molecule and three electron pairs are bonded with three hydrogen. (ie, one lone pair is present). 3 bp and 1 lp.
Hence, this molecule posses an irregular geometry (distortion from tetrahedral) Pyramidal.
Ex. 3: H2O
Step -1
Number of valence electron of Oxygen (central atom) = 6
Number of bonded atoms (two hydrogen) = 2
Total = 8
Step - 2
divide this by 2 (8/2) = 4
so there must be 4 electron pairs in this molecule and two electron pairs are bonded with two hydrogen. (ie, two lone pairs are present). 2 bp and 2 lp.
Hence, this molecule posses an irregular geometry (distortion from tetrahedral) bent shaped.
Ex. 4: BrF5
Step -1
Number of valence electron of bromine (central atom) = 7
Number of bonded atoms (five fluorine) = 5
Total = 12
Step - 2
divide this by 2 (12/2) = 6
so there must be 6 electron pairs in this molecule, but only five electron pairs are bonded with five fluorine atoms. (ie, one lone pair is present). 5 bp and 1 lp.
Hence, this molecule is distorted from octahedral geometry (irregular geometry) and is square pyramidal in shape.
We can use the above calculation for predicting the shape of ions also.
Ex -5: NH+4 (ammonium ion)
Step -1
Number of valence electron of nitrogen (central atom) = 5
Number of bonded atoms (three hydrogen) = 4
Number of positive charge (one) subtract 1 (loss of e-) = -1
Total = 8
Step - 2
divide this by 2 (8/2) = 4
so there must be 4 electron pairs in this molecule and four electron pairs are bonded with four hydrogen. (ie, no lone pair is present). 4 bp and 0 lp.
Hence, this molecule posses an regular geometry tetrahedral. (Compare this with ammonia)
For acquiring a positive charge one electron must be removed. Hence, in the above example we subtract 1(one). Similarly for negative ions we have to add electrons in calculation.
For molecules which have oxygen atom doubly bonded with central atom (ex. CO2, SO3) the following formula gives the expected geometry.
Number of electron pairs = 1/2{V+(n x v)}-(3 x n)
V - valance electrons of central atom
n - number of bonded atoms
v - valance electrons of bonded atom
EX - 6: CO2
Number of electron pairs = 1/2{4+(2x6)}-(3 x 2)
= 1/2{4 + 12} - (6)
= 1/2{16} - 6
= 8 -6
= 2
There are 2 electron pairs are present and both are bonded with oxygen hence it is linear.