Bonding in compounds using Molecular Orbital theory is explained in a basic level in this post. Examples for both homonuclear and heteronuclear diatomic molecules with appropriate MO diagrams are also illustrated for easy understanding. Hope this will help you while preparing your university examinations.
MOLECULAR ORBITAL THEORY:
Valance bond theory was unable to explain the magnetic behavior of oxygen and the non-existence of helium molecule. Hence another theory called " Molecular OrbitalTheory" was developed through the efforts ofFriedrich Hund, Robert Mulliken, John C. Slater, and John Lennard-Jones.
Features of Molecular orbital Theory:
1. When two atomic orbitals overlap, two new orbitals called as Molecular orbitals, designated as bonding and anti bonding are formed.
2. Molecular Orbitals give the electron distribution around a group of nuclei.
3. Molecular orbitals are formed only when the energy and the orientation of the two atomic orbitals involved are comparable (equal).
4. The number of MOs formed is equal to the number of combining AOs.
σ
5. The BMO has lower energy and greater energy than the ABMO.
6.The filling of electrons in MOs takes place according to the same rules as those of AOs.
Differences between Molecular Orbital and Atomic Orbital
ATOMIC ORBITALS:
1. It consists of one nucleus.
2. The electrons are under the influence of one nucleus.
3.AOs are represented by s, p, d and f.
4. They have simple shapes.
MOLECULAR ORBITALS.
1. It consists of more than one nucleus.
2. Electrons are under the influence of more than one nucleus.
3. MOs are represented by σ, σ* Π and Π*
4.They have complex shapes.
According to the Molecular Orbital Theory, individual atoms combine to form molecular orbitals. Electrons in a molecule occupy molecular orbitals. We can obtain the wave function of a molecular orbital by the following methods.
- Linear Combination of Atomic Orbitals (LCAO)
- United Atom Method
Linear Combination of Atomic Orbitals (LCAO)
According to this method, the formation of molecular orbitals is due to the Linear Combination (addition or subtraction) of atomic orbitals which combine to form the molecule.
Bonding Molecular Orbitals
Consider two atoms A and B which have atomic orbitals described by the wave functions ΨA and ΨB.When the two wave functions add together , one type of molecular orbitals formed are Bonding Molecular Orbitals. We can represent them by
ΨMO = ΨA + ΨB.
They have lower energy than atomic orbitals involved.
Anti-Bonding Molecular Orbitals
When molecular orbital forms by the subtraction of wave function, the type of molecular orbitals formed are antibonding Molecular Orbitals. We can Which can be represented as
ΨMO = ΨA – ΨB.
They have higher energy than atomic orbitals.
Hence, the combination of two atomic orbitals results in the formation of two molecular orbitals. They are the bonding molecular orbital (BMO) and the anti-bonding molecular orbital (ABMO).
Rules for Filling of Molecular Orbitals
The same rules used to fill atomic orbitals can be used while filling the molecular orbitals.
1. Aufbau Principle – molecular orbital which have the lowest energy are filled first.
2. Pauli’s Exclusion Principle – each molecular orbital can accommodate maximum of two electrons having opposite spins.
3. Hund’s Rule – in two molecular orbitals of the same energy, the pairing of electrons will occur when each orbital of same energy consist one electron.
Energy of various molecular orbitals is as follows:
Using Spectroscopy, the energy levels of these molecular orbitals were determined.
For O2 and higher molecules →
σ1s, σ *1s, σ 2s, σ *2s, σ 2pz, [π2px = π2py], [π*2px= π*2py], σ *2pz
For N2 and lower molecules →
σ 1s, σ *1s, σ 2s, σ *2s, [π 2px = π 2py], σ 2px [π *2px= π *2py], σ*2pz
Bond Order
The number of bonds between a pair of atoms is called the bond order.It may be calculated as the half of difference between the number of electrons present in the bonding orbitals and the antibonding orbitals that is,
Bond order (B.O.) = (No. of electrons in BMO - No. of electrons in ABMO)/ 2
Magnetic Behavior:
If all the molecular orbitals in species are spin paired, the substance is diamagnetic. But if one or more molecular orbitals are singly occupied it is paramagnetic.
Molecular Orbital Diagrams
This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram.
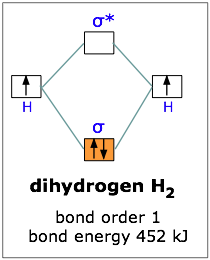
Example: 1
H2 molecule
Hydroden atom has one electorn in its 1s orbital. In the formation of Hydrogen molecule, the two atomic orbitals of each hydrogen combines to form two molecular orbital (BO and ABMO). The two Electrons are added to molecular orbitals associated lowest energy. or bonding, molecular orbital, as shown in the figure below.
The above diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms. As a result, the H2 molecule is more stable than a pair of isolated atoms.
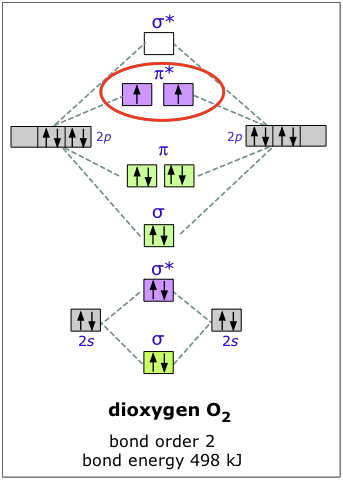
Example 2:
oxygen molecule
The electron configuration of oxygen is 1s22s22p4. In O2, therefore, we need to accommodate twelve valence electrons (six from each oxygen atom) in molecular orbitals. The Following MO diagram shows the filling of electrons in various MOs.
There are two unpaired electrons in the oxygen molecule, Hence it is paramagnetic.
Heteronuclear diatomic molecule
- Usually, atomic orbitals with energy levels similar to each other will overlap to form molecular orbitals. In the case of heteronuclear diatomic molecules, the atomic orbitals having energies which are close to each other will combine to form bonding and anti-bonding MOs. The mismatched (unequal) energy orbitals remain as non-bonding orbitals.
- There is an electronegativity difference between the two atoms in heteronuclear diatomic molecules. This causes the electrons to attract them towards the atom with the greater electronegativity. Thus the molecular orbital diagram will no longer be symmetric. Instead, the more electronegative element is drawn lower in energy and contributes more to the bonding orbital. And the less electronegative element is drawn at a higher energy level and contributes more to the antibonding orbital.
Lets take HF molecule, and construct MO diagram by applying the above mentioned points.
Example: 3
Hydrogen Fluoride
1. The 1s and 2s orbitals of Fluorine are so low in energy compared to the 1s orbital in Hydrogen, that they cannot be combined to form MOs.
2.The three 2p orbitals of Fluorine have close energy to combine with 1s orbital in Hydrogen.
3.Of the three 2p orbitals, the 2px and 2py orbitals of Fluorine have an insignificant spatial overlap (wrong orientation) with the 1s orbital in Hydrogen that they also do not form MOs.
4.Only the 2pz orbital of Fluorine has significant overlap with the 1s orbital in Hydrogen and can mix with it energetically Producing BO and ABMO.
5.Thus the MOs in HF are formed by the combination of 1s of H and 2pz of F atomic orbitals. This produces one BO and one ABMO.
6.The eight valance electrons ( 7 from F and 1 from H) of HF molecule fill all the orbitals except anti-bonding orbital as shown in the diagram below.
Note :
nb - non- bonding,  - bonding MO,
- bonding MO,  - anti-bonding MO.
- anti-bonding MO.
7.The
Bond order of HF is 1. It is
diamagnetic.
Comparison of VBT and MOT.
Points of Similarity:
According to both theories,
1. A covalent bond is formed by the result of orbital overlap.
2. For bond formation, the overlapping AOs must be nearly the same energy and same symmetry about the molecular axis.
3. Directional character of a covalent bond is explained.
Points of Difference:
1. VBT considers only the valance electrons involved in bond formation while MOT considers all the electrons in bond formation.
2. Role of ionic terms in the wave function is taken into account only by MO theory.
3. Resonance play an important role in VBT.
References:
https://www.emedicalprep.com/study-material/chemistry/chemical-bonding/molecular-orbital-theory/
https://www.toppr.com/guides/chemistry/chemical-bonding-and-molecular-structure/molecular-orbital-theory/
https://chem.libretexts.org
http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_15/node1.html
![\includegraphics[scale=0.75]{HF_correlation.eps}](https://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_15/img76.png)



