In this post, valence bond theory of covalent bonding is discussed in a basic level. small videos are also posted to understand atomic orbital overlapping concept and hybridization concept.
VALENCE BOND THEORY
The valence bond theory states that "atoms in a covalent bond share electron density through the overlapping (ஒன்றுடன் ஒன்று படிதல்)of their valence atomic orbitals".This creates an area of electron pair density between the two atoms.
EXAMPLE: Hydrogen molecule
The 1s orbitals of the two hydrogens approach each other and overlap to form a bond known as a sigma bond (σ bond) It is also known as s-s overlapping.
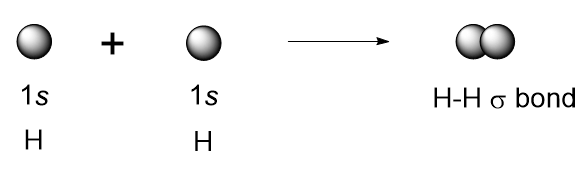
(1) an orbital on one atom overlaps an orbital on a second atom and
(2) the single electrons in each orbital combine to form an electron pair.
Figure below shows the energy changes of H2 molecule when bonding occurs.
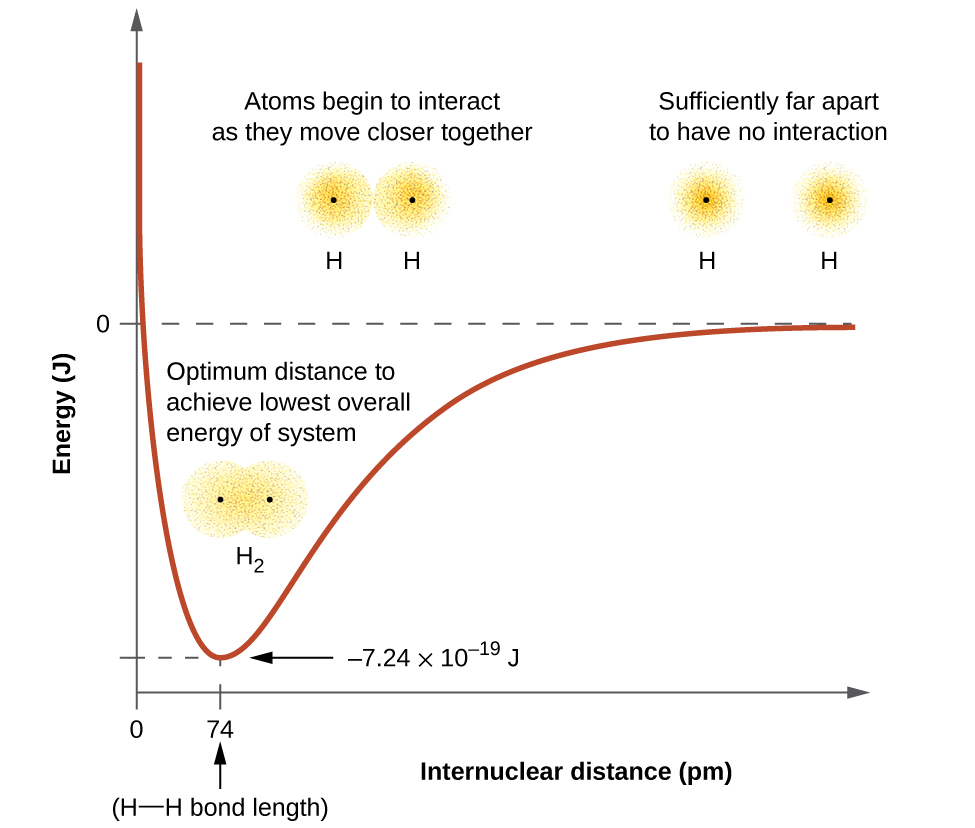
(a) The interaction of two hydrogen atoms changes as a function of distance. (b) The energy of the system changes as the
the atoms interact. The lowest (most stable) energy occurs at a distance of 74 pm, which is the bond length observed for the H2 molecule.
Similarly a s atomic orbital with a p atomic orbital overlaps to form sigma bond (s-p overlaping). Example: Hydrogen fluoride (HF)
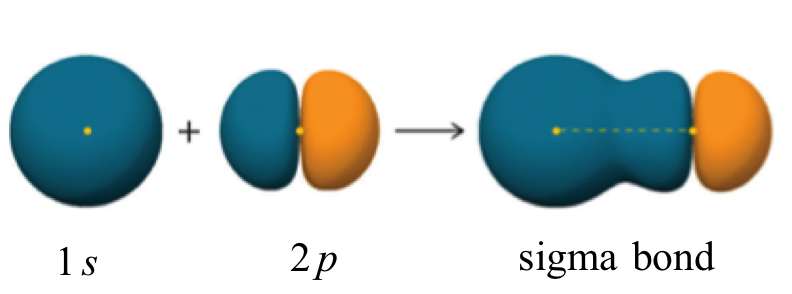
A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, F2, is a sigma bond formed by the overlap of two half-filled 2p orbitals, one from each fluorine atom.

References:
1. https://opentextbc.ca/introductorychemistry/chapter/valence-bond-theory-and-hybrid-orbitals-2/
2. https://opentextbc.ca/chemistry/chapter/8-1-valence-bond-theory/#CNX_Chem_08_01_sigma
3. https://chem.libretexts.org
4.https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Hybrid_Orbitals
5.http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch02/ch2-3.html
the atoms interact. The lowest (most stable) energy occurs at a distance of 74 pm, which is the bond length observed for the H2 molecule.
Similarly a s atomic orbital with a p atomic orbital overlaps to form sigma bond (s-p overlaping). Example: Hydrogen fluoride (HF)

A sigma bond can also be formed by the overlap of two p orbitals. The covalent bond in molecular fluorine, F2, is a sigma bond formed by the overlap of two half-filled 2p orbitals, one from each fluorine atom.

HYBRIDIZATION
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them.
The concept involves the "cross breeding" of atomic orbitals to create "new" orbitals. Hence the use of the term "hybrid" : think of a hybrid animal which is a cross breed of two species.
The intermixing of
two or more pure atomic orbitals of an atom with almost same
energy to
give same number of identical and degenerate new type of orbitals is
known as hybridization. The
new orbitals formed are also known as hybrid orbitals.

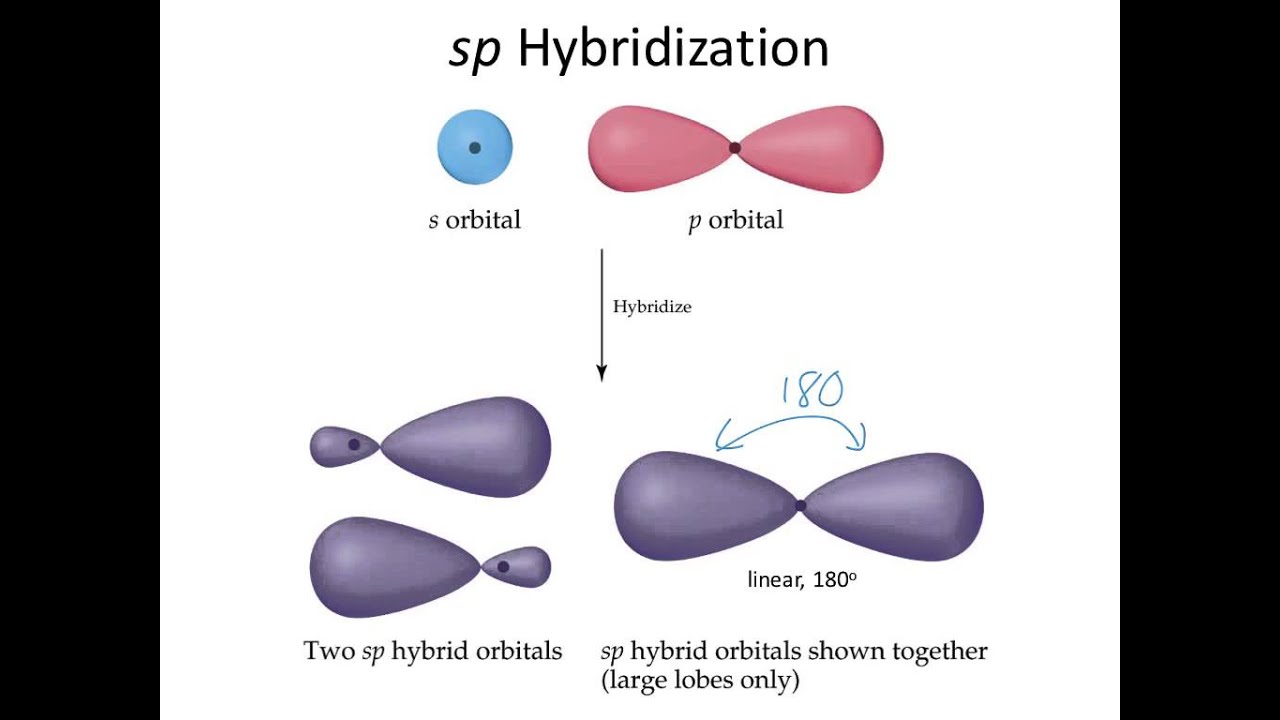
The above diagram shows a sp-hybrid orbital formed by inter-mixing of s and p atomic orbital. Look the two newly formed hybrid orbitals are identical in shape. The sp hybrid orbitals are arranged linearly (180 degree). They posses 50% s and 50% p character.
Example: Formation of BeCl2 using sp-hybridization is shown in the following video.
1. https://opentextbc.ca/introductorychemistry/chapter/valence-bond-theory-and-hybrid-orbitals-2/
2. https://opentextbc.ca/chemistry/chapter/8-1-valence-bond-theory/#CNX_Chem_08_01_sigma
3. https://chem.libretexts.org
4.https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Fundamentals/Hybrid_Orbitals
5.http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch02/ch2-3.html



No comments:
Post a Comment