Before we are going to discuss about other examples of hybridization, the first part of this post deals with how to present your answers in our university examination regarding the questions on hybridization concept .
Q: What is sp2 hybridization? Explain the formation of BF3 molecule.
Ans:
Mixing of one pure 's' and two pure 'p' orbitals to form three new 'sp2 ' hybrid orbitals of nearly equal energy is known as sp2 hybridization. This hybridization leads to trigonal planar geometry.
Characteristics:
- 1. All three hybrid orbitals are equivalent in shape and energy.
- 2. Three hybrid orbitals lie in the same plane and are directed towards three corners of an equilateral triangle.
- 3. Bond angle is 1200.
Formation of BF3 molecule
The central boron atom has the following electronic configuration.
B: 1s2,2s2,2p1
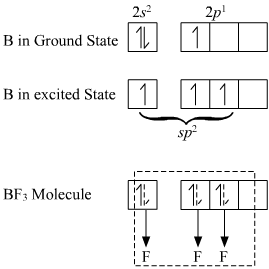
In the formation of BF3, one of the electron from completely filled 2s orbital is promoted to 2p orbital to give the following excited electronic state configuration.
B*: 1s2,2s1,2px12py1
Now the three singly filled orbitals hybridize to (inter mixing) give three sp2 hybrid orbitals which are identical in shape and in energy. These three sp2 hybrid orbitals are pointed towards the three corners of an equilateral triangle. The three sp2 hybrid orbitals overlaps with the singly filled p orbitals of three fluorine atoms t o give BF3 molecule.
EXAMPLE :
BF3

A view of sp2 hybrid orbital.


Diagram showing the overlapping of sp2 hybrid orbitals of B with 2P orbital of F to form BF3 molecule
Intermixing of one 's'and three 'p'orbitals of almost equal energy to form four identical and degenerate sp3 hybrid orbitals is called sp3 hybridiztion. The four sp3 hybrid orbitals are oriented in tetrahedral symmetry at an angle of 109o.28'. It has 25% s character and 75% p character.
EXAMPLE:
CH4


Diagram showing the sp3 hybridization in methane.
NOTE:
The above format may be followed to explain other types of hybridization.
SP2 and SP3 hybridization
sp2 hybridization
Intermixing of one
's' and two 'p' orbitals of almost equal energy to give three identical and degenerate
hybrid orbitals is known as sp2
hybridization. The three sp2 hybrid orbitals are oriented in trigonal planar symmetry at an angle of120O to each other. The sp2
hybrid orbitals have 33.3% 's' character and 66.6%'p'character.
EXAMPLE :
BF3

A view of sp2 hybrid orbital.


Diagram showing the overlapping of sp2 hybrid orbitals of B with 2P orbital of F to form BF3 molecule
SP3 Hybridization
Intermixing of one 's'and three 'p'orbitals of almost equal energy to form four identical and degenerate sp3 hybrid orbitals is called sp3 hybridiztion. The four sp3 hybrid orbitals are oriented in tetrahedral symmetry at an angle of 109o.28'. It has 25% s character and 75% p character.
EXAMPLE:
CH4


Diagram showing the sp3 hybridization in methane.



No comments:
Post a Comment