The remaining types of hybridization are discussed in this post with clear pictures and animated videos. Hope this will make you understand better.
sp3d hybridization :
The mixing of one s ,three p and one d-atomic orbitals to form five sp3d hybrid orbitals of equal energy is called sp3d hybridization
Example: PCl5
The ground state electronic configuration of phosphorus atom is: 1s2 2s22p6 3s23px13py13pz1.
Phosphorus atom undergoes excitation to promote one electron from 3s orbital to one of empty 3d orbital.
Thus the electronic configuration of 'P' in the excited state is 1s2 2s22p6 3s13px13py13pz1 3d1.
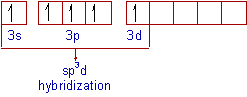
Intermixing of half - filled one 3s, three 3p and one 3dz orbitals to give five sp3d hybrid orbitals. The shape of the the orbitals participating in sp3d hybridization are shown below.
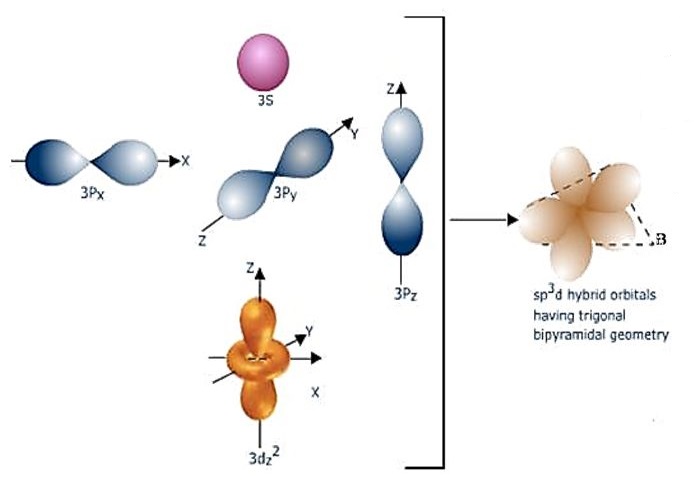
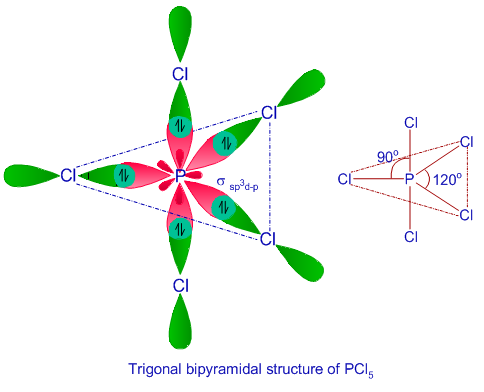
The sp3d hybrid orbitals which are arranged in trigonal bipyramidal symmetry are shown in the diagram below.
IMPORTANT:
The five orbitals in sp3d hybridizationare not equivalent. These are divided into two sets :
a) Equatorial hybrid orbitals :
Three hybrid orbitals are directed towards the corners of an equilateral triangle are called equatorial hybrid orbitals. These are planar and bond angle is 1200.
b) Axial hybrid orbitals :
Two hybrid orbitals are perpendicular to the plane of equatorial hybrid orbital .These are called axial hybrid orbital . They make an angle of 900 with equatorial hybrid orbitals.
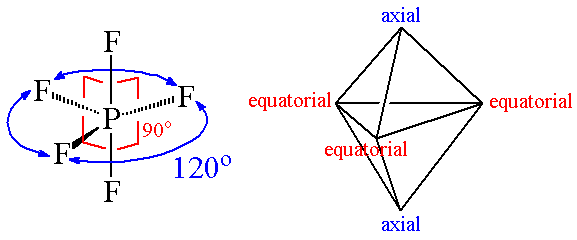
Diagram showing equatorial and axial bonds in PCl5.
Geometry : trigonal bipyramidal.
Bond angle is 900 and 1200.
sp3d2 hybridization :
The mixing of one s, three p and two d- atomic orbitals to form six equivalent sp3d2 hybrid orbitals of equal energy. This hybridization is known as sp3d2 hybridization.
The electronic configuration of 'S' in ground state is 1s2 2s22p6 3s23px23py13pz1.
Promotion of two electrons (one from 3s and one from 3px)into two of the 3d orbitals gives the excited state configuration of 1s2 2s22p6 3s13px13py13pz13d2.
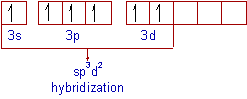
In the excited state, sulfur under goes sp3d2 hybridization by mixing a 3s, three 3p and two 3d orbitals. Thus formed six half filled sp3d2 hybrid orbitals are arranged in octahedral symmetry.
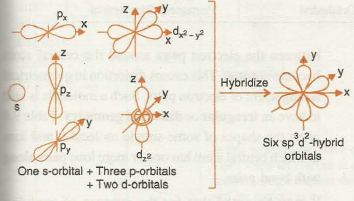
Diagram showing the shape of orbitals involved in sp3d2 hybridization
In SF6, four out of six hybrid orbitals are lying in one plane while remaining two are directed above and below the plane containing four hybrid orbitals perpendicularly.
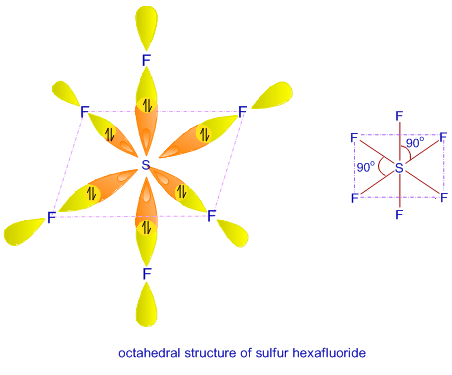
Geometry: octahedral
Bond angle: 900.
Sp3d3 hybridization :
The mixing of one s, three p and three d- atomic orbitals to form seven equivalent sp3d3hybrid orbitals of equal energy. This hybridization is known as sp3d3 hybridization.
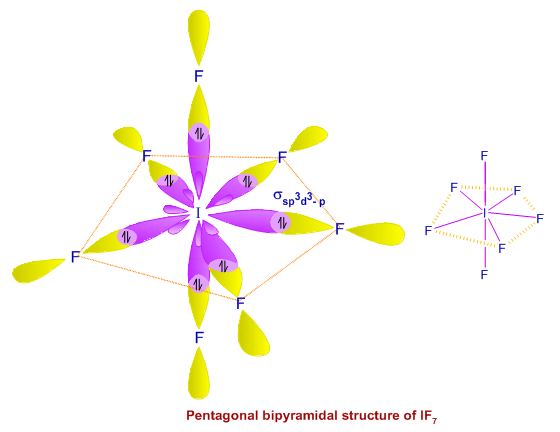
Example : IF7
The electronic configuration of Iodine atom in the ground state is: [Kr]4d105s25p5. Since the formation of IF7 , the iodine atom promotes three of its electrons (one from 5s orbital and two from 5p sublevel) into empty 5d orbitals.
The electronic configuration of Iodine in the excited state can be written as: [Kr]4d105s15p35d3.
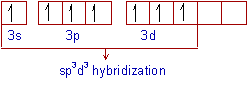
In the excited state, iodine atom undergoes sp3d3 hybridization to give 7 half filled sp3d3 hybrid orbitals in pentagonal bipyramidal symmetry. These will form 7 σsp3d3-p bonds with fluorine atom. Diagram below shows the orbitals involved in the formation of IF7.
Geometry: pentagonal bipyramidal
Bond angle: 720 and 900.
Courtesy:
https://chemistryonline.guru/hybridization.
https://www.adichemistry.com/general/chemicalbond/vbt/hybridization-illustrations.html





No comments:
Post a Comment